Sublingual Treatment for Migraine & Cluster Headaches
Abort or prevent vascular headaches such as migraine, migraine variants, or so called "histaminic cephalagia".
This page is intended for US healthcare professionals only
The Only FDA Approved Sublingulal Treatment
Ergomar® (Ergotamine) is an ergot medication that treats certain types of headaches, including cluster headaches and migraines by tightening blood vessels and calming certain parts of the brain to relieve headache pain.
Prescribing Ergomar®
Ergomar® is easy to prescribe and provides considerable patient savings to eligible commercially insured patients
Patients may pay as little as $0* for Ergomar®
The Ergomar® Savings Program offers eligible patients up to $800 savings on a month supply of Ergomar® (up to 20 tablets per month).
No patient registration is required to use the card
The savings card can be applied to initial prescriptions, refills, and 30 or 90-day prescriptions

How Ergomar® Works
Ergomar® is an alpha adrenergic blocking agent that helps narrow blood vessels, reduces vasoconstrictive effects of vascular headaches, and has a rapid onset of action (vasoconstriction occurs in as little as 15 minutes).
As a sublingual, Ergomar® dissolves under the tongue, entering the blood stream from tissues beneath the tongue, bypasses the gastrointestinal tract and first-pass metabolism, and makes self-medication easier and increases patient compliance.



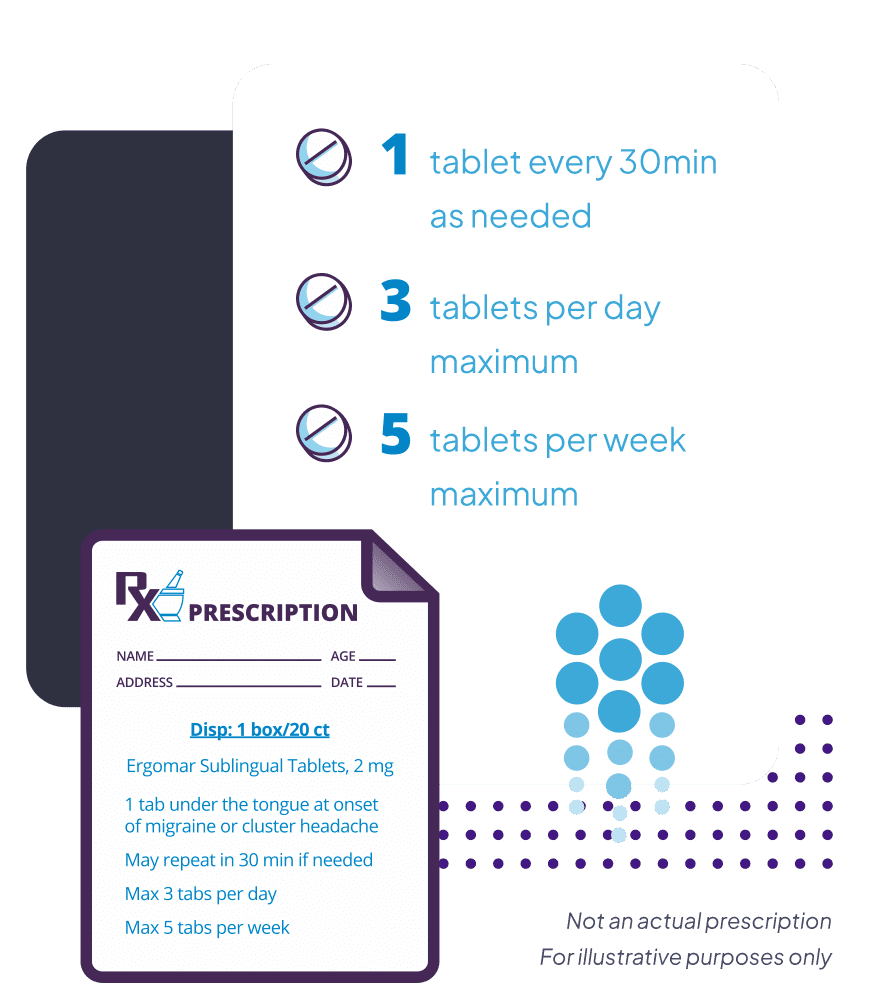
1
Tablet every 30 minutes
3
Tablets per a day maximum
5
Tablets per a week maximum
Additional Medical Information; Rx only
Important Warning
Serious and/or life-threatening peripheral ischemia has been associated with the coadministration of ergotamine tartrate with potent CYP 3A4 inhibitors including protease inhibitors and macrolide antibiotics. Because CYP 3A4 inhibition elevates the serum levels of ergotamine tartrate, the risk for vasospasm leading to cerebral ischemia and/or ischemia of the extremities is increased. Hence, concomitant use of these medications is contraindicated.
Indications & Usage
Ergomar® is indicated as therapy to abort or prevent vascular headache, e.g., migraine, migraine variants or so-called "histaminic cephalalgia."
Please see Full Prescribing Information, including Boxed Warning, and Important Safety Information on the product itself.
Individual patient results may vary. To report suspected adverse reactions, contact the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. You may also contact Pangea Pharmaceuticals at 855-892-8224 or drugsafety@pangeapharm.com.
How Supplied
Ergomar® Sublingual Tablets are round, green tablets each containing 2 mg of ergotamine tartrate. They are debossed with the product identification code "LB2" on one side, and are supplied in unit-dose cartons of 20 tablets (10 tablets x 2 cards), NDC 81279-104-20 (discontinued former NDC 70720-120-20).
Contraindications
Do not administer Ergomar® with potent CYP 3A4 inhibitors such as protease inhibitors or macrolide antibiotics as this has been associated with acute ergot toxicity (ergotism) characterized by vasospasm and ischemia of the extremities, with some cases resulting in amputation. There have been rare reports of cerebral ischemia in patients on protease inhibitors when ergotamine was coadministered, at least one resulting in death.
Ergomar® Sublingual Tablets should not be administered to pregnant women, nursing mothers or women who may become pregnant.
Ergomar® Sublingual Tablets should not be given to patients with peripheral vascular disease, coronary heart disease, hypertension, impaired hepatic or renal function or sepsis.
Ergomar® Sublingual Tablets should not be given to patients with hypersensitivity to ergotamine.
Warnings & Precautions
Coadministration of ergotamine with potent CYP 3A4 inhibitors such as protease inhibitors or macrolide antibiotics has been associated with serious adverse events; for this reason, these drugs should not be given concomitantly with ergotamine. While these reactions have not been reported with less-potent CYP 3A4 inhibitors, there is a potential risk for serious toxicity including vasospasm when these drugs are used with ergotamine.
There have been a few reports of patients on ergotamine tartrate and caffeine therapy developing retroperitoneal and/or pleuropulmonary fibrosis. There have also been rare reports of fibrotic thickening of the aortic, mitral, tricuspid, and/or pulmonary valves with long-term continuous use of ergotamine tartrate and caffeine.
Ergomar® Sublingual Tablets should not be administered with other vasoconstrictors. Nicotine may provoke vasoconstriction in some patients, predisposing to a greater ischemic response to ergot therapy.
While most cases of ergotism associated with ergotamine treatment result from frank overdosage, some cases have involved apparent hypersensitivity. Care should be exercised to ensure that the patient stays within the limits of recommended dosage.
In rare instances, patients may display withdrawal symptoms consisting of rebound headache upon discontinuation of treatment.
There have been reports of drug abuse and psychological dependence in patients on ergotamine tartrate therapy.
Ergomar® Sublingual Tablets should not be used for chronic daily administration. Total daily dosage must not exceed three tablets in any 24-hour period. Total weekly dosage should not exceed five tablets (10 mg) in any one week.
Adverse Reactions
Cardiovascular Vasoconstrictive complications of a serious nature may occur at times. These include ischemia, cyanosis, absence of pulse, cold extremities, gangrene, precordial distress and pain, EKG changes and muscle pains. Although these effects occur most commonly with long-term therapy at relatively high doses, they have also been reported with short-term or normal doses. Other cardiovascular adverse effects include transient tachycardia or bradycardia and hypertension.
Gastrointestinal: Nausea and vomiting.
Neurological: Paresthesias, numbness, weakness and vertigo.
Allergic: Localized edema and itching.
Fibrotic Complications: (See PRECAUTIONS)
For complete prescribing and dosage information click here